Abstract

Background: Patients with essential thrombocythemia (ET) remain at risk of significant thrombotic complications, despite use of validated risk stratification and treatment pathways. Procoagulant platelets, a platelet subpopulation which supports thrombin generation, are elevated in prothrombotic conditions. We hypothesize that ET patients exhibit high procoagulant platelet potential and the procoagulant platelet subset may be a biomarker for risk of poor disease outcomes, enabling identification of patients for therapeutic escalation.
Methods: 70 ET patients, 20 polycythemia vera (PV) patients and 32 age-matched healthy controls were recruited at Concord Hospital, Australia and Singapore General Hospital (development cohort). Outcome data including thrombosis, death and transformation to myelofibrosis and acute leukemia were prospectively collected. Procoagulant platelets were measured by uptake of a tripeptide trivalent arsenical (GSAO) combined with P-selectin expression by flow cytometry, with or without stimulation with increasing thrombin concentrations (0.5-5 U/mL) ± 10 µg/mL collagen, in whole blood samples. a Flow cytometry data were analyzed using both standard and a multiparameter clustering approach (FlowSOM) with FlowJo software. A hypergate algorithm based on fluorescence intensity of six parameters was developed using R programming language to enumerate a novel GSAO+ platelet subpopulation in each patient flow cytometry dataset. Time to event analysis was performed on cohorts defined by receiver operating characteristics (ROC) analysis identified cut-off for the subpopulation, with clinician adjudication of outcomes blinded to biomarker data. 25 consecutive ET patients were subsequently recruited from Concord Hospital outpatient clinics in a prospective validation study for an independent time to event analysis.
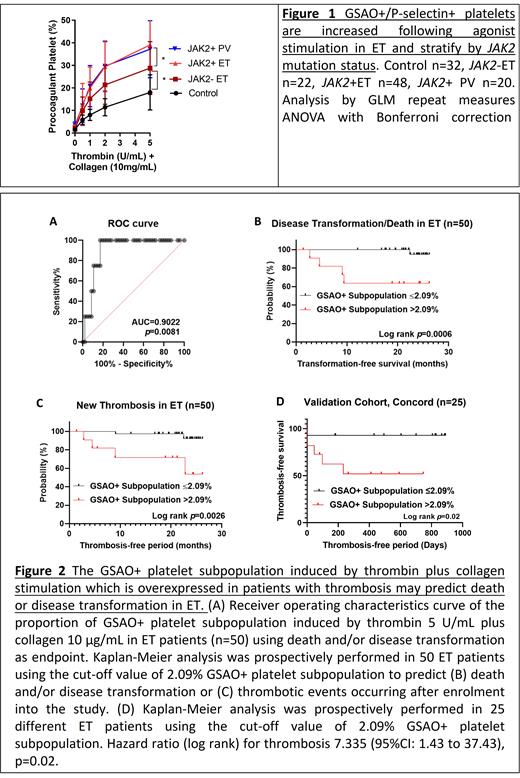
Results: ET (n=70) and PV (n=20) patients in the development cohort demonstrated marked increased GSAO+/P-selectin+ procoagulant response to thrombin±collagen compared to healthy controls (n=32), segregating according to JAK2 V617F mutation status (Figure 1). Multivariate analysis (age, sex, thrombosis history, cardiovascular risk factors, hydroxyurea, platelet and leukocyte counts and mutation status) demonstrated that only male sex (p=0.013) and JAK2 V617F mutation (p=0.004) were independent predictors of thrombin response in ET. FlowSOM analysis identified a high GSAO+ platelet subpopulation significantly overexpressed in ET patients with thrombosis history (p=0.001). A ROC curve to evaluate the high GSAO+ platelet subpopulation as a predictor of disease transformation and/or death yielded a higher predictive value than the total GSAO+/P-selectin+ population (AUC of 0.9022, p=0.0081, Figure 2A). Prospective follow up with time to event analysis (development cohort) showed a ROC derived cut-off of 2.09% GSAO+ subpopulation at time of recruitment (100% sensitivity, 73.9% specificity) was predictive of death and/or disease transformation (p=0.0006, Figure 2B) or new thrombotic events (p=0.0026, Figure 2C) in patients already on primary or secondary prevention therapy. In the validation cohort, time to event analysis (n=25, median time to census 20 months, range 2.7-30) demonstrated a hazard ratio of 7.3 (95% CI: 1.4 to 37.4, p=0.02, Figure 2D) for new thrombotic events with GSAO+ subpopulation of >2.09%, independent of potential covariates (mutation status, IPSET score, historical thrombosis, anti-platelet therapy). In 3 of 3 patients in which biomarker bloods were collected at clinical review for thrombosis prior to initiation of therapy, the GSAO+ subpopulation was markedly increased and then reduced following disease modification therapy.
Conclusion: This study, including validation in a small prospective cohort with pre-specified outcomes, identifies a high GSAO+ platelet flow cytometry subpopulation as a potential novel risk stratification marker for transformation and/or recurrent thrombotic events in ET. The biomarker can be collected at multiple timepoints during a patient's clinical course for dynamic risk assessment and early data suggests utility in monitoring response to therapy. Larger multisite studies to evaluate the hypergate performance as a biomarker for poor outcomes in patients with ET appear warranted.
aPasalic et al. J Thromb Haemost. 2018 Jun;16(6):1198-1210.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal